Trace of the Interesting "V"-Shaped Dynamic Mechanism of Interactions between Water and Ionic Liquids
Citation
Bingjie Sun, Qiu Jin, Lisha Tan, Peiyi Wu*, and Feng Yan. Trace of the Interesting "V"-Shaped Dynamic Mechanism of Interactions between Water and Ionic Liquids. J. Phys. Chem. B 2008, 112, 14251-14259.
Abstract
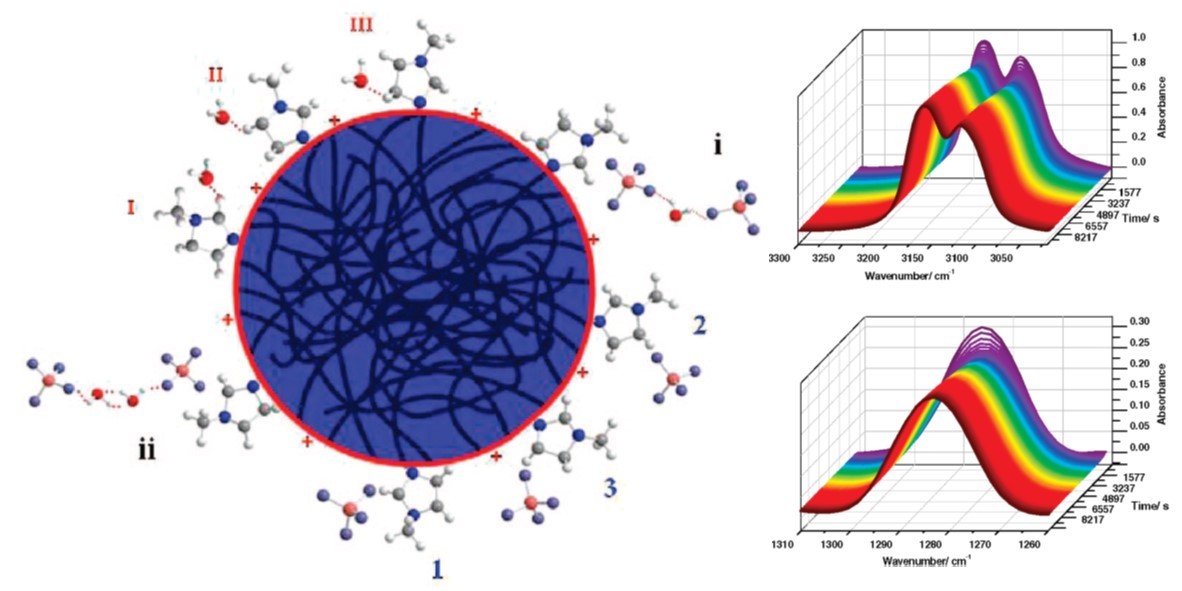
The mixture of ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate, bmimBF4) and water (2.5%, molar fraction) under isothermal conditions at 80 °C was investigated by FTIR spectroscopy and two-dimensional correlation infrared spectroscopy (2D-IR) methods. Three regions were focused: the OH stretching band of water (3755−3300 cm−1), the stretching band of CH on the imidazole ring (3300−3020 cm−1), and the BF stretching band of anions (1310−1260 cm−1). During this process, water was gradually evaporated as time passed, which produced influences on the interactions among cations, anions, and water molecules. In the FTIR analysis, we found an interesting “V”-shaped changing trend in peak areas of the C−H on the imidazole ring and the B−F stretching band; the inflection of the system was 913 s, gained through the “moving window” method. A two-step variation was accordingly found during this process. Hydrogen bonds formed by water molecules with cations or water molecules with anions were destroyed by the reduction of water, making a fall in the former period of “V” process, while electrostatic interactions newly formed between anions and cations leading to a rise during the latter period of this course. In this paper, various conformations formed among cations, anions, and water molecules were clearly assigned, and we managed to trace the whole dynamic mechanism of this isothermal process by 2D-IR techniques.