Thermally Induced Dissociation Nature of Pure 2-Pyrrolidinone Via Near-Infrared Correlation Spectroscopy Analysis
Citation
Hui Tang, Shengtong Sun, and Peiyi Wu*. Thermally Induced Dissociation Nature of Pure 2-Pyrrolidinone Via Near-Infrared Correlation Spectroscopy Analysis. Appl. Spectrosc. 2009, 63, 1174-1180.
Abstract
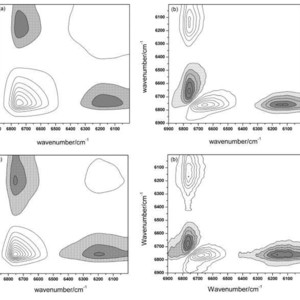
The temperature-dependent dissociation behavior of 2-pyrrolidinone in the pure liquid state was investigated via perturbation-correlation moving-window two-dimensional (PCMW2D) correlation spectroscopy and two-dimensional near-infrared (2D-NIR) correlation spectroscopy. Absorption bands in the region of 6900–6000 cm−1 assigned to the first overtones of stretching modes of NH groups provided detailed information about the dissociation process from 25 °C to 95 °C. On the basis of PCMW2D analysis, the sequence of dissociation events occurring during the temperature variation were elucidated in two temperature ranges (25–60 °C and 65–95 °C). Specific dissociation order of the hydrogen bond under temperature perturbation was disclosed by 2D-NIR correlation spectroscopy. As temperature increased, the dissociation of larger oligomers occurred firstly with the generation of smaller oligomers, which were then consumed later by the decomposition of hydrogen bonds. The dissociation of stable cyclic dimers occurred mainly at higher temperature through gradually weakened hydrogen-bonding of NH groups.