Trace of the Thermally Induced Evolution Mechanism of Interactions Between Water and Ionic Liquids
Citation
Bingjie Sun, and Peiyi Wu*. Trace of the Thermally Induced Evolution Mechanism of Interactions between Water and Ionic Liquids. J. Phys. Chem. B 2010, 114, 9209-9219.
Abstract
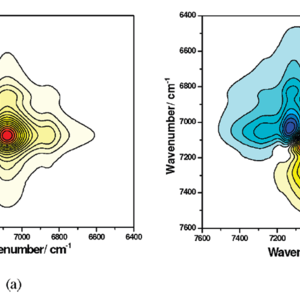
The thermally induced evolution mechanisms of various interactions in ionic liquids (1-butyl-3-methylimidazolium tetrafluoroborate, bmimBF4) and water mixtures have been investigated in this paper. In the near-infrared (NIR) spectroscopy, we focus mainly on ν(OH) and ν(CH) overtone regions. During heating of bmimBF4 and water mixtures, the ν(OH) overtone peak shows a significant blue shift, and the area of this peak shows different changes in three heating regions. By using the perturbation correlation moving window (PCMW) method, we have ascertained the critical temperatures of these three regions: 25−100, 105−160, and 165−190 °C, and we have accordingly performed 2D correlation NIR analysis in three parts. On the basis of 2D study results, we find several types of O−H involved hydrogen bonds (H-B’s) in a bmimBF4 and water (15 mol %) mixture and arrive at their evolution mechanisms in each heating region. During heating at 25−100 °C, strong H-B’s, such as BF4−···water···BF4− and BF4−···cyclic water dimer···BF4−, transform into weaker H-B’s with simpler structures; at 105−160 °C, the remaining BF4−···water···BF4− continues to dissociate, and cation···water H-B’s start to dissociate, and a large amount of released free water evaporates; whereas in the final heating region of 165−190 °C, BF4−···water···BF4− still exists and continues to dissociate, and in the study of the ν(CH) overtone region, we have found that the concentration of water in bmimBF4 affects interactions between cations and anions. In the mixture of bmimBF4 with more water (15 mol %), H-B’s between water and bmimBF4 cannot be completely destroyed, even at very high temperature; therefore, only limited new electrostatic interactions would be formed between cations and anions during heating, but in a mixture of bmimBF4 with less water (15 mol %), cations and anions are able to form new electrostatic interactions during the heating process. However, the intensity of these interactions is smaller than that in the 80 °C isothermal process due to the low contacting possibilities among ions at high temperatures.