Aqueous Solutions of Poly(ethylene oxide)-Poly(N-isopropylacrylamide): Thermosensitive Behavior and Distinct Multiple...
Abstract
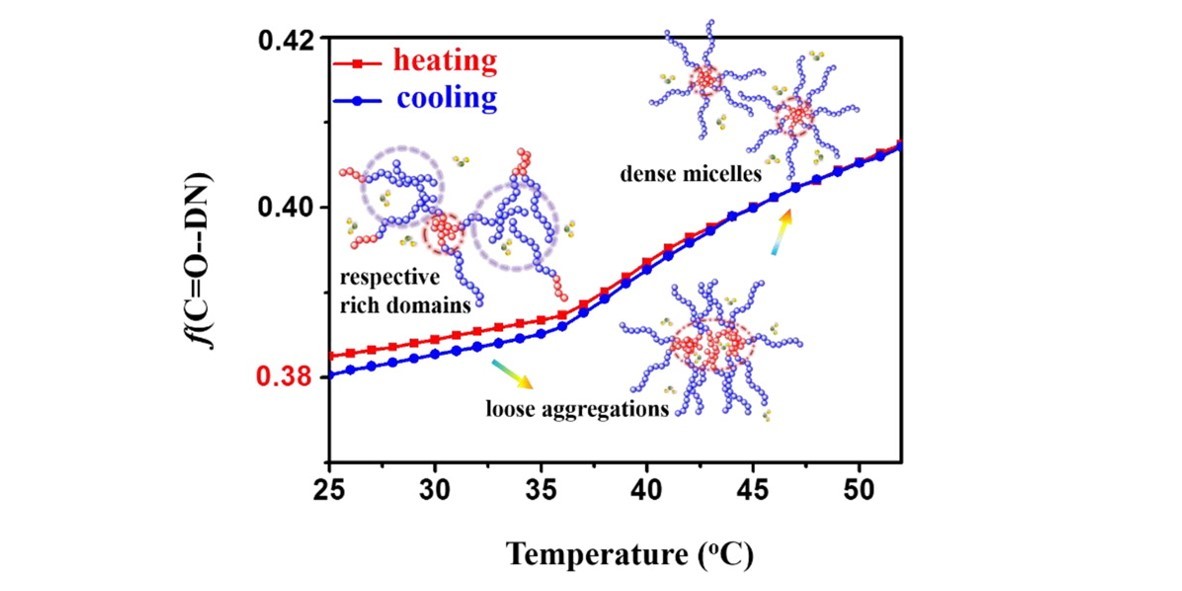
Detailed phase transition and conformational changes taking place as a function of temperature in poly(ethylene oxide)-b-poly(N-isopropylacrylamide) (PEO-b-PNIPAM) semidiluted aqueous solutions are elucidated in the present study. By the use of elaborate vibrational spectroscopy techniques in combination with two-dimensional correlation spectroscopy (2Dcos), three transition regions including respective rich domains (<29 °C), loose aggregations (30–36 °C), and dense sphere micelles (>37 °C) are depicted. In particular, subtle variations of hydrogen bonds are detected even under the lower critical solution temperature (LCST), and the respective rich domain regime is marked with strong participation from hydrogen bonding at different concentrations and compositions. Both the formation of intermolecular hydrogen bonds and the less hydration degrees of PNIPAM segments compared with PNIPAM homopolymer at elevated temperatures verify the evolution of PNIPAM from their own domains to loose aggregations with PEO shells. Dense micelles are formed beyond the LCST of PNIPAM, while the outmost PEOs act as buffer layers and postpone the shrinkage of PNIPAM chains. Due to the existence of a buffer layer, higher phase transition temperatures compared with PNIPAM homopolymer are observed.
<<全文链接>>

