Molecular Evolution of Poly(2-isopropyl-2-oxazoline) Aqueous Solution during the Liquid–Liquid Phase Separation...
Abstract
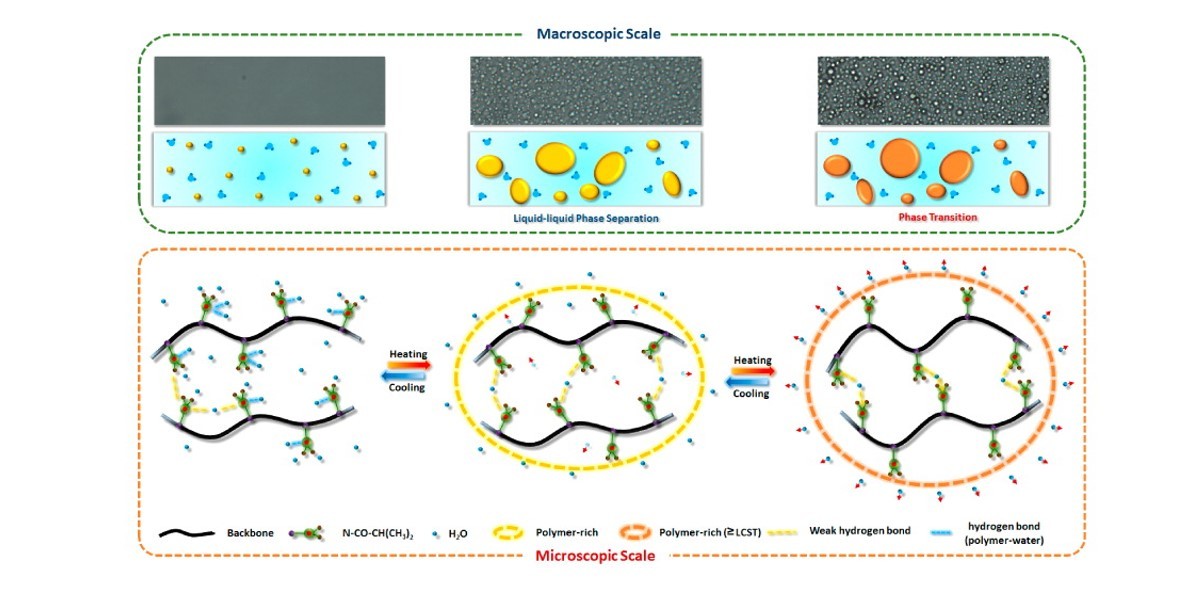
A detailed phase transition process of poly(2-isopropyl-2-oxazoline) (PIPOZ) in aqueous solution was investigated by means of DSC, temperature-variable 1H NMR, Raman, optical micrographs, and FT-IR spectroscopy measurements. Gradual phase separation accompanied by large dehydration degree and big conformational changes above the lower critical solution temperature (LCST) and facile reversibility were identified. Based on the two-dimensional correlation (2Dcos) and perturbation correlation moving window (PCMW) analyses, the sequence order of chemical group motions in phase transition process was elucidated. Additionally, a newly assigned CH3···O═C intermolecular hydrogen bond at 3008 cm–1 in the PIPOZ system provides extra information on the interactions between C–H and C═O groups. The formation of cross-linking “bridging” hydrogen bonds C═O···D–O–D···O═C (1631 cm–1) is proposed as the key process to induce the liquid–liquid phase separation and polymer-rich phase formation of PIPOZ solution. With slow heating, more and more “bridging” hydrogen bonds were formed and D2O were expelled with an ordered and mostly all-trans conformation adopted in the PIPOZ chains. On the basis of these observations, a physical picture on the molecular evolution of PIPOZ solution during the phase transition process has been derived.
<<全文链接>>

