Exploration of Doubly Thermal Phase Transition Process of PDEGA-b-PDMA-b-PVCL in Water
Abstract
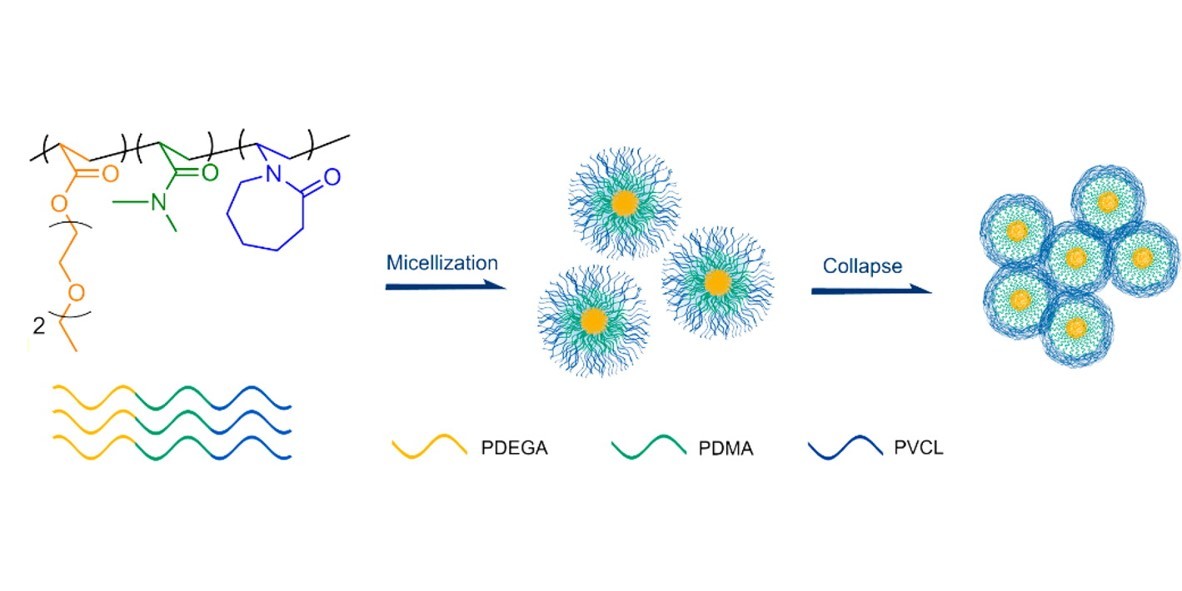
Understanding of phase transition mechanism of thermoresponsive polymers is the basis for the rational design of smart materials with predictable properties. Linear ABC triblock terpolymer poly(di(ethylene glycol)ethyl ether acrylate)-b-poly(N,N-dimethylacrylamide)-b-poly(N-vinylcaprolactam) (PDEGA-b-PDMA-b-PVCL) was synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization. The doubly thermal phase transition of PDEGA-b-PDMA-b-PVCL in aqueous solution was investigated by a combination of nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC), turbidimetry, and dynamic light scattering (DLS). The terpolymer self-assembles into micelles with PDEGA being the core-forming block during the first lower critical solution temperature (LCST) transition corresponding to PDEGA, which is followed by a second LCST transition corresponding to PVCL, resulting in the formation of micellar aggregates. The PDMA middle segment plays an important role as an isolation zone to prevent cooperative dehydration of the PDEGA and PVCL segments, and therefore, two independent LCST transitions corresponding to PDEGA and PVCL were observed. Furthermore, FT-IR with perturbation correlation moving window (PCMW) and two-dimensional spectroscopy (2DCOS) was applied to elucidate the two-step phase transition mechanism of this terpolymer. It was observed that the CH, ester carbonyl, and ether groups of PDEGA change prior to the CH and amide carbonyl groups of PVCL, further supporting that the two phase transitions corresponding to PDEGA and PVCL indeed occur without mutual interferences.
<<全文链接>>

