Microdynamic Changes of Moisture-Induced Crystallization of Amorphous Calcium Carbonate...
Citation
Meng Cheng, Shengtong Sun*, and Peiyi Wu*. Microdynamic Changes of Moisture-Induced Crystallization of Amorphous Calcium Carbonate Revealed by in-Situ FTIR Spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 21882-21889.
Abstract
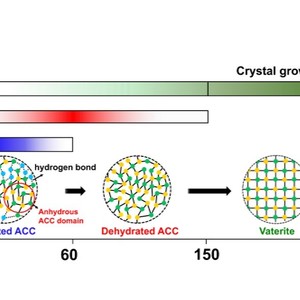
Amorphous calcium carbonate (ACC) is the most important intermediate phase in the nucleation/crystallization process of CaCO3, and thus the proper interpretation of how ACC transforms into final crystals at the molecular level, is crucial to understand various biomineralization phenomena. Herein, we successfully monitored the moisture-induced crystallization process of ACC via in-situ FTIR spectroscopy, which is very sensitive to the specific changes of the different vibrational modes of carbonates and water molecules. In combination with the tools of perturbation correlation moving window and two-dimensional correlation spectroscopy, it is found that the driving force of ACC crystallization is the fracture of hydrogen bond formed by H2O···CO32-. The bending vibrations of carbonate are more sensitive to moisture permeation than stretching modes, and the whole crystallization process can be divided into three sequential stages, i.e., the hydrated ACC firstly loses its structural water and converts to the dehydrated ACC, which then gradually transforms into vaterite, followed by the final growth of vaterite crystals. Anhydrous ACC microdomains are found to be already existing in the as-prepared ACCs.