The Influence of a Thermoresponsive Polymer on the Microdynamic Phase Transition Mechanisms...
Citation
Lan Ma, Ge Wang, Shengtong Sun*, and Peiyi Wu*. The Influence of a Thermoresponsive Polymer on the Microdynamic Phase Transition Mechanisms of Distinctly Structured Thermoresponsive Ionic Liquids. Phys. Chem. Chem. Phys. 2017, 19, 22263-22271.
Abstract
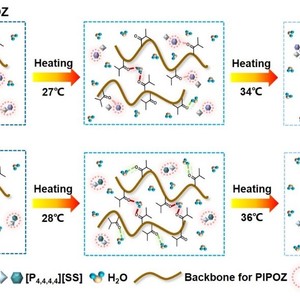
The study of a ternary solution involving a thermoresponsive polymer, a thermoresponsive ionic liquid (IL), and a solvent will not only help with interpreting their distinct phase transition behavior, but also promote the development of novel thermoresponsive systems. In this paper, we investigate the influence of a conventional thermoresponsive polymer, poly(2-isopropyl-2-oxazoline) (PIPOZ), on the phase transition behavior of two thermoresponsive ILs ([P4,4,4,6][MC3S], [P4,4,4,4][SS]) with different structures. Although the addition of PIPOZ reduces the transition temperatures of both ILs, our analyses demonstrate that there exists large difference in the microdynamic phase transition mechanisms between [P4,4,4,6][MC3S]/PIPOZ and [P4,4,4,4][SS]/PIPOZ aqueous solutions. Both PIPOZ and [P4,4,4,6][MC3S] experience unexpectedly an unusual over-hydration process before a two-step phase transition of the mixture solution, which can be explained by the presence of a new kind of intermolecular bridging hydrogen bond (IL-water-polymer), while only PIPOZ undergoes dehydration around the transition temperature of [P4,4,4,4][SS]/PIPOZ aqueous solution. Further spectral analyses reveal that both [P4,4,4,6][MC3S] and PIPOZ engage in the phase separation of the ternary solution jointly, while PIPOZ takes part in the phase transition of [P4,4,4,4][SS]/PIPOZ solution more independently.