UCST or LCST? Composition-Dependent Thermoresponsive Behavior of Poly(N-acryloylglycinamide-co-diacetone acrylamide)
Abstract
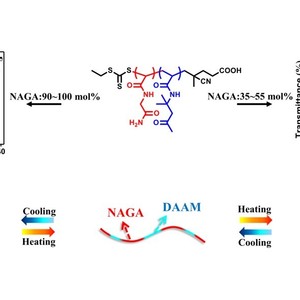
Copolymerization has been widely used to tune the thermoresponsive behavior of water-soluble polymers. However, the observation of both upper and lower critical solution temperature (UCST and LCST) from the same type of copolymer comprising only one monomer whose homopolymer is thermosensitive and the other monomer whose homopolymer is nonthermosensitive has not been reported. In this work, well-defined thermoresponsive copolymers with tunable compositions have been synthesized by copolymerization of N-acryloylglycinamide (NAGA) and diacetone acrylamide (DAAM) via reversible addition–fragmentation chain transfer (RAFT) polymerization. The thermal transitions of these copolymers are investigated using a combination of turbidimetry, dynamic light scattering (DLS), proton nuclear magnetic resonance (1H NMR), and Fourier transform infrared (FTIR) spectroscopy. The solubility of these copolymers shows a distinct dependence on the composition. While copolymers with up to 30 mol % NAGA are essentially insoluble, copolymers with 35–55 mol % NAGA or 90–100 mol % NAGA have either LCST- or UCST-type transitions respectively, and soluble copolymers are obtained with 60–85 mol % NAGA. The LCST- and UCST-type transitions are tunable with respect to composition, degree of polymerization, polymer concentration, isotope effect and the presence of electrolyte. Insights from variable-temperature 1H NMR and FTIR spectroscopies reveal the key role of hydrogen-bonding between the NAGA and DAAM units in determining the thermal transitions.

<<全文链接>>