A Two-Dimensional Correlation ATR-FTIR Study of Poly(vinyl methyl ether) Water Solution
Citation
Yilu Guo, Yun Peng, and Peiyi Wu*. A Two-Dimensional Correlation ATR-FTIR Study of Poly(vinyl methyl ether) Water Solution. J. Mol. Struct. 2008, 875, 486-492.
Abstract
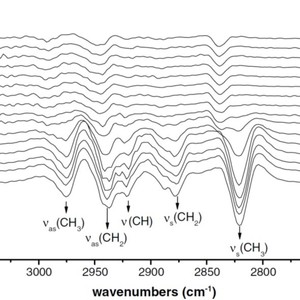
Thermosensitive phase transition behavior of poly(vinyl methyl ether) (PVME) in an aqueous solution during heating was investigated by Fourier transform infrared (FTIR) spectroscopy with attenuated total reflection (ATR) accessory. ATR-IR spectra of PVME in an aqueous solution change dramatically in the vicinity of the lower critical solution temperature (LCST). Heating of the solution above LCST leads to the lower wavenumber shifts of C–H stretching bands, which result from the hydration of polymer chains. Two-dimensional infrared (2D IR) analysis results indicate that hydrated C–H groups change prior to dehydrated C–H groups with increasing temperature around the phase transition. Furthermore, the CH2 groups in the main chain dehydrate more slowly than the side chain methyl groups. When 0.5 M KCl was added into a PVME aqueous solution, IR spectra showed that the phase transition temperature was reduced, and the phase separation proceeded in two successive steps. On the other hand, features of 2D IR spectra did not change compared to PVME aqueous solution in the absence of KCl. The result indicates that the underlying phase transition mechanism itself was not altered by the presence of KCl, although the transition temperature is shifted.