A Infrared Spectroscopic Study on the Mechanism of Temperature-Induced Phase Transition of Concentrated ...
Citation
Hengjie Lai, and Peiyi Wu*. A Infrared Spectroscopic Study on the Mechanism of Temperature-Induced Phase Transition of Concentrated Aqueous Solutions of Poly(N-isopropylacrylamide) and N-Isopropylpropionamide. Polymer 2010, 51, 1404-1412.
Abstract
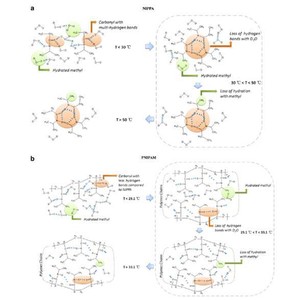
FTIR in combination with perturbation correlation moving window (PCMW) technique was applied to study the phase transition of concentrated aqueous solutions of Poly(N-isopropylacrylamide) (PNIPAM) and its small molecular model compound N-isopropylpropionamide(NIPPA). It was found that lower critical solution temperature (LSCT) of 40% NIPPA/D2O solution was 39 °C which was higher by ca. 8 °C than that of PNIPAM, and that NIPPA exhibited much wider temperature ranges of phase transition from 30 to 50 °C while PNIPAM underwent the phase separation in a narrow temperature range (29.1–33.1 °C). Moreover, we utilized two-dimensional correlation infrared spectroscopy (2DIR) analysis to reveal that the presence of main chains didn’t affect the sensitivity and changing sequence of different groups, but did have a strong effect on the size of aggregation and formation of hydrogen bonds between carbonyl groups and water molecules. Without the interference of hydrophobic main chains, the carbonyls of NIPPA (1600 cm−1) could interact with more water than those of PNIPAM (1627 cm−1) below LSCT, which was the reason of the slower and milder phase transition taking place in NIPPA system.