Interchain Hydrogen Bonding Interaction Induced Phase Behaviors of PEO-b-PVK/PAA Blend
Abstract
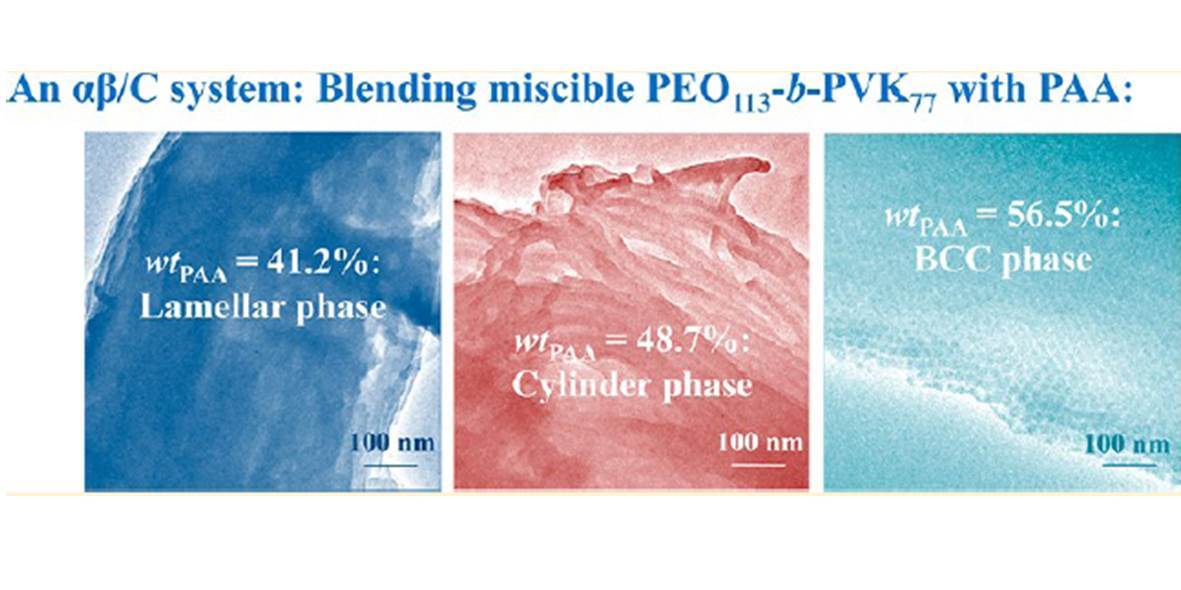
In this paper, we study an αβ/C blend system composed of a diblcok copolymer of poly(ethylene oxide)113-b-poly(N-vinylcarbazole)77 (PEO113-b-PVK77) and a homopolymer of poly(acrylic acid) (PAA) with a weight-average molecular weight of 1900 g/mol. The phase behavior of the blend was characterized using various techniques including Fourier transform infrared spectroscopy, differential scanning calorimetry, small-angle X-ray scattering, and transmission electron microscopy. The experimental results indicate that while the PEO and PVK blocks are miscible in bulk state, adding PAA into the diblock can lead to microphase separation of the blend due to the interchain hydrogen bonding complexation between PEO and PAA. The phase morphology of the blend is strongly dependent on the PAA composition. Only with sufficiently high weight fraction of PAA (wtPAA), the blends can present ordered microphase-separated structures, although the long-range order is not well developed. For 40% < wtPAA < 70% (corresponding to the PVK volume fraction between 44% and 22%), the ordered phase is changed from lamella to cylinder to cubic phase with increasing wtPAA. Further increase of PAA content results in spheres with the core of PVK dispersed in the matrix of PAA. The diameters of the PVK cores of cylinders and spheres are very close to the twice of the unperturbed end-to-end distance of the PVK block, indicating that the rather rigid PVK block is hard to be deformed during microphase separation. The PAA composition dependence of the blend phase behavior illustrates that the degree of PEO/PAA complexation dominates the segregation strength. After the microphase separation occurs, the phase morphology can be adjusted by varying the PAA content.
<<全文链接>>

