Hydration Capabilities and Structures of Carbonyl and Ether Groups in Poly(3-(2-methoxyethyl)-N-vinyl-2-pyrrolidone)...
Abstract
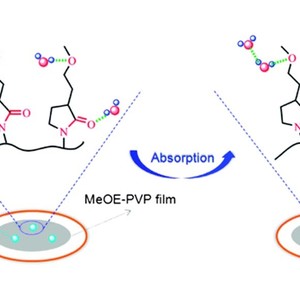
Poly(3-(2-methoxyethyl)-N-vinyl-2-pyrrolidone) (MeOE-PVP), a potential material in the pharmaceutical industry, is an amphiphilic polymer with hydrophobic CH groups and two different hydrophilic groups (C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O and C–O), and readily absorbs water vapor from humid environments. In this work, the hydration capabilities and structures of different chemical groups in MeOE-PVP film during water vapor sorption and desorption process are investigated by in situ FTIR spectroscopy, two-dimensional (2D) correlation analysis and density functional theory (DFT) calculations. When the film is tested in a stable humid environment, the changes of the C
O and C–O), and readily absorbs water vapor from humid environments. In this work, the hydration capabilities and structures of different chemical groups in MeOE-PVP film during water vapor sorption and desorption process are investigated by in situ FTIR spectroscopy, two-dimensional (2D) correlation analysis and density functional theory (DFT) calculations. When the film is tested in a stable humid environment, the changes of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O group are more sensitive to water vapor than those of the C–O group, which is further verified by 2DIR maps, with much more hydrated structures of the C
O group are more sensitive to water vapor than those of the C–O group, which is further verified by 2DIR maps, with much more hydrated structures of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O group being formed after moisture absorption. Compared with MeOE-PVP in aqueous solution, it is believed that C–O group is a relatively stable hydrophilic group from which is difficult to lose water molecules once it is at the hydrated state. In addition, combined with DFT calculations, it is observed that C
O group being formed after moisture absorption. Compared with MeOE-PVP in aqueous solution, it is believed that C–O group is a relatively stable hydrophilic group from which is difficult to lose water molecules once it is at the hydrated state. In addition, combined with DFT calculations, it is observed that C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O and C–O groups of MeOE-PVP are inclined to interact with water molecules in the form of CO⋯H2O and CO⋯H2O⋯H2O, but as CO⋯(H2O)2, probably due to limited absorption concentration and contact area in the MeOE-PVP film.
O and C–O groups of MeOE-PVP are inclined to interact with water molecules in the form of CO⋯H2O and CO⋯H2O⋯H2O, but as CO⋯(H2O)2, probably due to limited absorption concentration and contact area in the MeOE-PVP film.

<<全文链接>>