In Depth Analysis on the Unusual Multistep Aggregation Process of Oligo(ethylene glycol) Methacrylate-Based Polymers...
Abstract
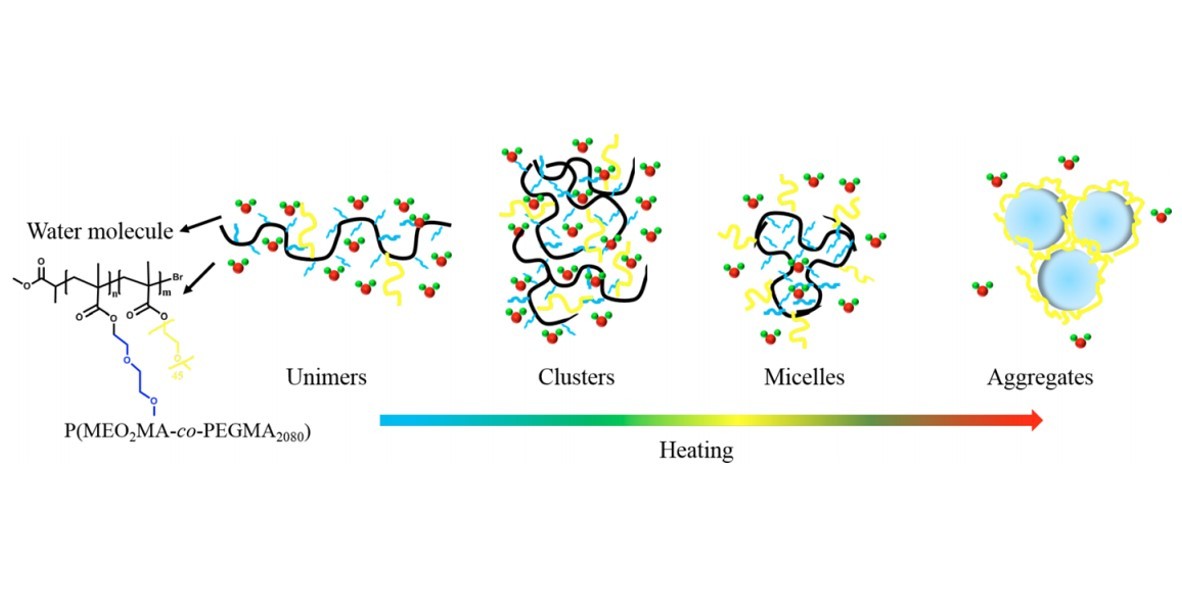
Dynamic thermal phase transition behavior of a thermoresponsive copolymer P(MEO2MA-co-PEGMA2080) synthesized by ATRP random copolymerization of 2-(2-methoxyethoxy)ethyl methacrylate(MEO2MA, Mn = 188 g/mol) and poly(ethylene glycol) methyl ether methacrylate (PEGMA, Mn= 2080 g/mol) in D2O was studied by means of infrared spectroscopy in combination with two-dimensional correlation analysis (2Dcos). Because of the absence of strong intermolecular hydrogen bonding interactions between polymer chains and the amphiphilic property of PEGMA2080 with long ethylene glycol segments, this copolymer exhibited an unusual thermally induced multistep aggregation process. In general, the change of hydrophilic side chain takes place before carbonyl groups and backbones during the whole process and thus the driving force of this phase transition process of P(MEO2MA-co-PEGMA2080) should be hydration changes of side chains. 2Dcos was finally employed to discern the sequence order of all the group motions during heating process in different temperature regions. It is concluded that during the phase transition P(MEO2MA-co-PEGMA2080) chains successively experience “unimers–clusters–micelles– aggregates” four consecutive conformation changes.
<<全文链接>>

