Two-Dimensional Correlation Infrared Spectroscopic Study on the Crystallization and Gelation of PVDF in Cyclohexanone
Citation
Yun Peng, Bingjie Sun, and Peiyi Wu*. Two-Dimensional Correlation Infrared Spectroscopic Study on the Crystallization and Gelation of Poly(vinylidene fluoride) in Cyclohexanone. Appl. Spectrosc. 2008, 62, 295-301.
Abstract
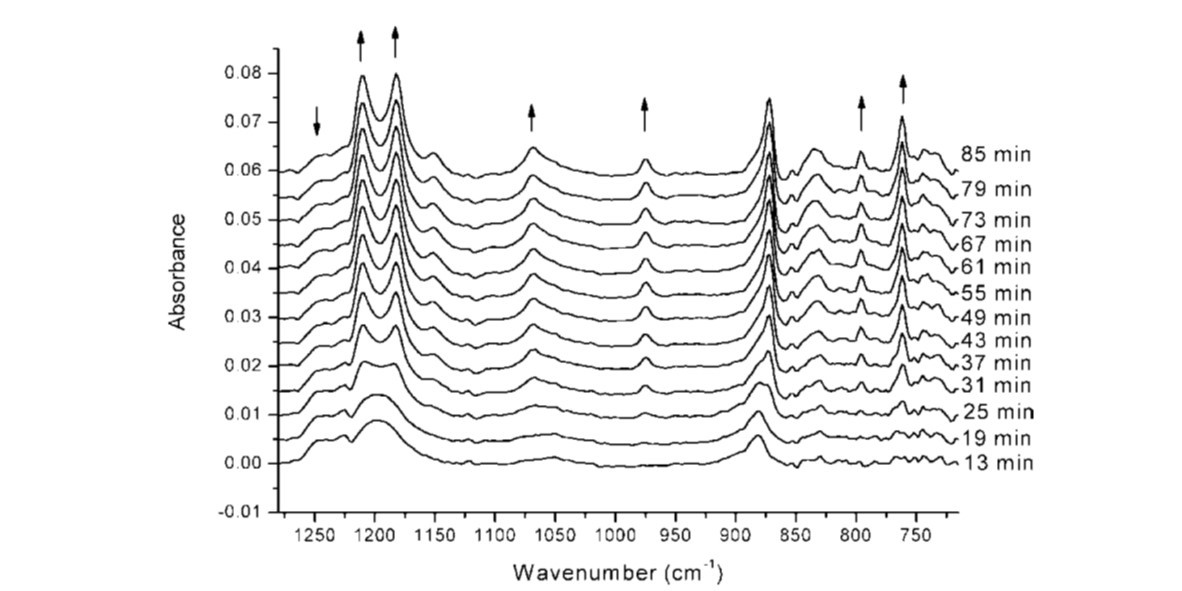
Poly(vinylidene fluoride) (PVDF) converts easily into a thermo-reversible gel through crystallization by standing at room temperature in cyclohexanone. In this study, the Fourier transform infrared (FT-IR) spectra were measured continuously at room temperature during the conversion of the solution into a gel. The IR difference spectra derived from these spectra by absorbance subtraction clearly indicate the presence of PVDF α-crystallites in the gel due to the presence of absorption bands corresponding to the TG+TG− conformation of the α-phase. In the time interval from 25 to 45 min after the beginning of the experiment, the IR bands of PVDF increased dramatically, indicating the conversion of polymer chains from random statistical coils to the ordered TG+TG− conformation (α-form). In the time interval from 45 to 90 min, the IR bands of PVDF increased slowly, reflecting no further crystallization. Using two-dimensional (2D) IR analysis, it could be shown that the v(C=O)) absorption band of cyclohexanone changed during the gelation process. During the conformational ordering process (25–45 min), the v(C=O) absorption band of the cyclohexanone dimer (1707 cm−1) decreased while the corresponding band of the monomer at 1718 cm−1 increased. Furthermore, a new band at 1695 cm−1 increased, which could be assigned to C=O groups of the solvent interacting with the CF2 groups in the polymer chain. The bands of the crystalline PVDF share positive cross-peaks with the bands of cyclohexanone, which indicates that the chain of PVDF changed prior to the cyclohexanone molecules during the conformational ordering process. However, these positive cross-peaks disappeared during the crystallization process, which means that the chain of PVDF changed synchronously with the solvent molecules. As for the bands of PVDF chains, the band at 762 cm−1 varied prior to the bands at 873 cm−1 and 796 cm−1 during the conformational ordering process. The 762 cm−1 absorption is assigned to the CF2 group of PVDF, the 873 cm−1absorption involves the C–C group of PVDF, and the 796 cm−1 band is attributed to the CH2groups of PVDF. Thus, the CF2 functionalities change faster than the C–C and CH2 groups. However, the correlation cross-peaks between 762 cm−1 and 873 cm−1 and at 796 cm−1disappeared during the later state of the gelation process. At the same time, the bands of PVDF and solvent still varied, which suggests that it is a physical interaction process between PVDF chain and solvent.