A FTIR and 2D-IR Spectroscopic Study on the Microdynamics Phase Separation Mechanism of the PNIPAM Aqueous Solution.
Citation
Bingjie Sun, Yinan Lin, Peiyi Wu*, and Heinz W. Siesler. A FTIR and 2D-IR Spectroscopic Study on the Microdynamics Phase Separation Mechanism of the Poly(N-isopropylacrylamide) Aqueous Solution. Macromolecules 2008, 41, 1512-1520.
Abstract
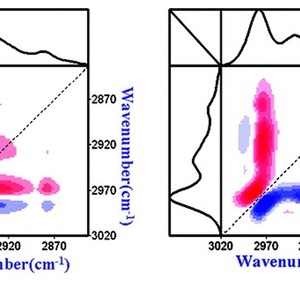
The thermal behavior of PNIPAM in its concentrated D2O solution (20 wt %) was studied by FTIR and 2D-IR correlation spectroscopy. The spectral data of the C−H groups and the Amide I region provide details about the changes of the hydrophobic and hydrophilic parts in the polymer respectively during a heating−cooling cycle. The reversal of peak positions of the C−H bands upon cooling indicates the reversibility of temperature-induced dehydration of the hydrophobic groups. The change in hydrogen bonding of C=O···D−N constructed between dehydrated C=O and N−D groups, as derived from the Amide I region, does not revert precisely in the cooling process due to the newly formed hydrogen bonds in the collapsed state, and a hysteresis phenomenon is observed. In our concentrated solution (20 wt %), the strength of those intra- and interchain hydrogen bonds even prevent the polymers from dissociating completely below the LCST during the cooling process. The microdynamics phase separation mechanism was obtained by application of the 2D-IR analysis to the spectral data. When the temperature rises, the two-step dehydration of the CH3 groups occurs first, then the main-chain diffusion and aggregation takes place, and finally the hydrogen bond transition occurs. The dynamic sequence in the cooling process is also described.