Volume Phase Transition Mechanism of Poly Oligo(ethylene glycol)methacrylate Based Thermo-Responsive Microgels...
Abstract
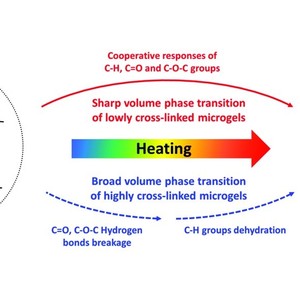
Thermo-dynamic volume phase transition mechanisms of poly[oligo(ethylene glycol)methacrylate] (POEGMA) based microgels with poly(ionic liquid) (PIL) cross-linking moieties are investigated in detail on the basis of temperature-dependent Fourier transform infrared (FTIR) spectroscopy. The original FTIR data are further analysed by two-dimensional correlation spectroscopy (2Dcos) with the perturbation correlation moving window (PCMW) technique. It is observed that the content of hydrophilic PIL cross-linking structure strongly affects the temperature induced volume phase transition mechanism of microgels in which the less cross-linked microgel exhibits a sharp volume phase transition process while the highly cross-linked microgel presents a broad transition behavior. Peculiarly, the dehydration of C–H groups acts as the driving force for the whole phase transition process within the less cross-linked microgel network and cooperative response of chemical groups is identified. It is deduced that the hydrophilic PIL moieties develop polymer–water–polymer interactions with C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups as C
O groups as C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O⋯D2O–PIL hydrogen bonds emerge in the less cross-linked system. As regards the highly cross-linked microgel system, the phase transition process is driven by the disruption of hydrogen bonds between C
O⋯D2O–PIL hydrogen bonds emerge in the less cross-linked system. As regards the highly cross-linked microgel system, the phase transition process is driven by the disruption of hydrogen bonds between C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups and water molecules while the response of C–H groups becomes insensitive. PIL moieties passively dehydrate following the dehydration of C–H groups on oligo(ethylene glycol) side chains and no hydrogen bond between C
O groups and water molecules while the response of C–H groups becomes insensitive. PIL moieties passively dehydrate following the dehydration of C–H groups on oligo(ethylene glycol) side chains and no hydrogen bond between C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O group and IL–D2O association appears during the phase transition process.
O group and IL–D2O association appears during the phase transition process.

<<全文链接>>