From globules to crystals: a spectral study of poly(2-isopropyl-2-oxazoline) crystallization in hot water
Abstract
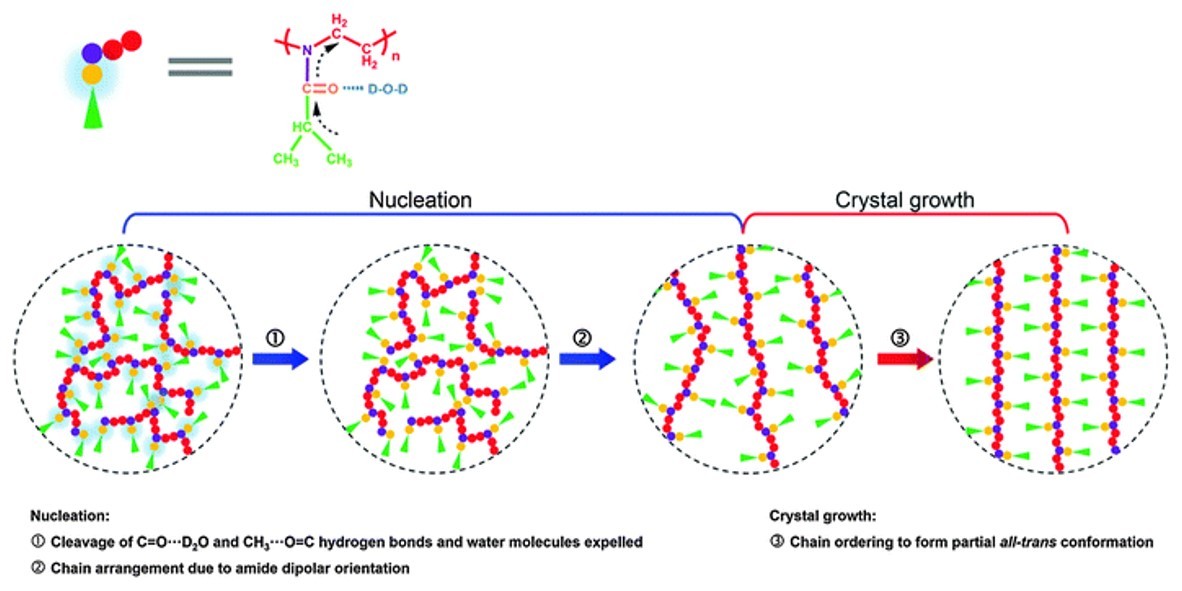
One easy strategy to comprehend the complex folding/crystallization behaviors of proteins is to study the self-assembly process of their synthetic polymeric analogues with similar properties owing to their simple structures and easy access to molecular design. Poly(2-isopropyl-2-oxazoline) (PIPOZ) is often regarded as an ideal pseudopeptide with similar two-step crystallization behavior to proteins, whose aqueous solution experiences successive lower critical solution temperature (LCST)-type liquid–liquid phase separation upon heating and irreversible crystallization when annealed above LCST for several hours. In this paper, by microscopic observations, IR and Raman spectroscopy in combination with 2D correlation analysis, we show that the second step of PIPOZ crystallization in hot water can be further divided into two apparent stages, i.e., nucleation and crystal growth, and perfect crystalline PIPOZ chains are found to only develop in the second stage. While all the groups exhibit changes in initial nucleation, only methylene groups on the backbone participate in the crystal growth stage. During nucleation, a group motion transfer is found from the side chain to the backbone, and nucleation is assumed to be mainly driven by the cleavage of bridging C=O⋯D–O–D⋯O=C hydrogen bonds followed by chain arrangement due to amide dipolar orientation. Nevertheless, during crystal growth, a further chain ordering process occurs resulting in the final formation of crystalline PIPOZ chains with partial trans conformation of backbones and alternative side chains on the two sides. The underlying crystallization mechanism of PIPOZ in hot water we present here may provide very useful information for understanding the crystallization of biomacromolecules in biological systems.
<<全文链接>>

