Spectral Insights into Gelation Microdynamics of N-Octyl-D-Gluconamide in Water
Abstract
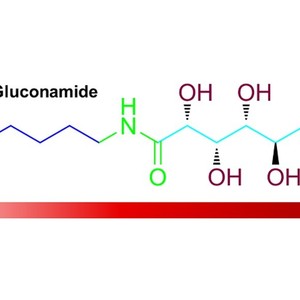
Near infrared spectroscopy in combination with two-dimensional correlation spectroscopy (2Dcos) and perturbation correlation moving window (PCMW) technique is employed to illustrate the gelation microdynamic mechanism of hydrogelator N-octyl-D-gluconamide (8-GA), which can rapidly self-agglomerate into helical bilayer micellar fibers upon cooling from spherical micelles. Boltzmann fitting and PCMW easily determined the gelation temperature to be ca. 72 °C and the transition temperature range to be 70–75 °C. Moreover, band shifting and splitting phenomena can be observed for CH-related overtones, indicating the formation of much ordered and tight hydrophobic core from octyl tails. On the other hand, 2Dcos was used to discern the sequential orders during the gelation process and concluded that all the group motions have a continuous transfer from the octyl tail to the chiral carbohydrate head followed by the final immobilization of the solvent, which meanwhile, is actually a continuous dehydration process from the hydrophobic core to the outer hydrophilic chiral head. The driving force of the gelation process in microdynamics can only be the dehydration process of hydrophobic octyl chains, but with final helical superstructures being stabilized by amide-associated hydrogen bonding and the “chiral bilayer effect” of carbohydrate heads.

<<全文链接>>